The Importance of Manufacturing Plant Documentation
ATS
SEPTEMBER 1, 2023
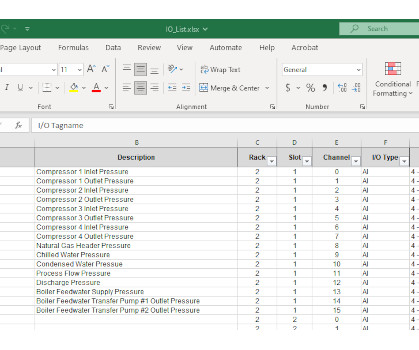
Share Plant documentation has long been a requirement for manufacturers, not only from a regulatory and compliance standpoint but also to support consistency, continuity and quality in operations and processes. Create initial documentation based on raw data. Create “final” documentation as part of the commissioning process.








































Let's personalize your content