QMS Software for Pharmaceutical Manufacturers
Snic Solutions
FEBRUARY 3, 2025
Discover practical steps for implementing a QMS in pharma to achieve quality excellence. Enhance compliance and operational efficiencyread more now!

Snic Solutions
FEBRUARY 3, 2025
Discover practical steps for implementing a QMS in pharma to achieve quality excellence. Enhance compliance and operational efficiencyread more now!
CMTC Manufacturing Tech
JULY 25, 2024
Reduces the Risk of Product Defects As a QMS Standard, ISO 13485 was designed to help medical device manufacturers and equipment suppliers develop strong quality management systems from the ground up. Additionally, MDSAP specifically facilitates market entry into countries like Australia, Brazil, Canada, Japan, and the U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MEM
JULY 20, 2023
.” PromoExQ MSC Growth Medium XF is manufactured under strict quality standards and in compliance with PromoCell’s EXCiPACT™ GMP certification scheme, which builds on the ISO 9001:2015 quality management system and allows the manufacturing of pharmaceutical excipients.

Manufacturer's Monthly
MAY 31, 2023
At the forefront of design excellence, ELGi was the first compressor company in the world to be certified with ISO 9001 for quality management systems in 1994, and in 2019 ELGi was the first and only global industrial air compressor manufacturer in over sixty years to be awarded the Deming Prize for excellence in Total Quality Management. “We

ATS
JULY 10, 2024
SOPs are often included within the Quality Management System (QMS). In industries like pharmaceuticals and food and beverage, where it’s especially important that proven methods and techniques are used, SOPs are mandatory. Beware of making it too long or too broad as this makes it harder to verify compliance.
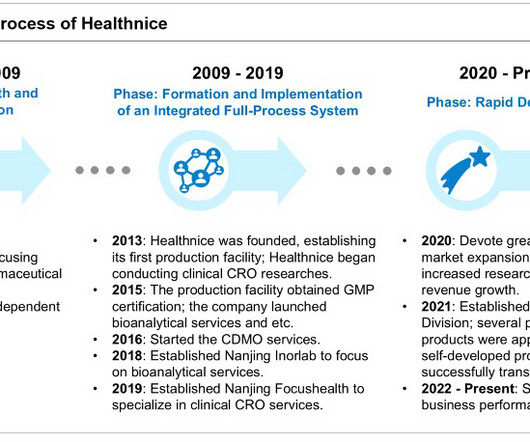
Frost & Sullivan Manufacturing
DECEMBER 25, 2024
Aligning with Industry Trends: Healthnice Advances Step by Step Amid the global pharmaceutical industry’s evolution, high-end preparations have become a mainstream trend in drug development. Therefore, for pharmaceutical companies, high-end preparations represent a high-value, cost-effective strategy for differentiation.
Let's personalize your content